To speed up computation of ![]() , electrostatic and van der Waals forces are commonly terminated at a specified distance (CTOFnb)
from the atom exerting the forces [12]. For van der Waals forces, use the CHARMM keyword VSHIft. For electrostatic forces,
the choice of approximation depends on the system to be simulated. Ewald summation is being used more and more as a way to treat
electrostatics without any cutoff in periodically repeated (infinite) systems such as solutions or crystals.
However, the appropriate choice of spherical cutoff
is still relevant for any modeling and simulation of a finite system, e.g., a hydrated protein in vacuum.
, electrostatic and van der Waals forces are commonly terminated at a specified distance (CTOFnb)
from the atom exerting the forces [12]. For van der Waals forces, use the CHARMM keyword VSHIft. For electrostatic forces,
the choice of approximation depends on the system to be simulated. Ewald summation is being used more and more as a way to treat
electrostatics without any cutoff in periodically repeated (infinite) systems such as solutions or crystals.
However, the appropriate choice of spherical cutoff
is still relevant for any modeling and simulation of a finite system, e.g., a hydrated protein in vacuum.
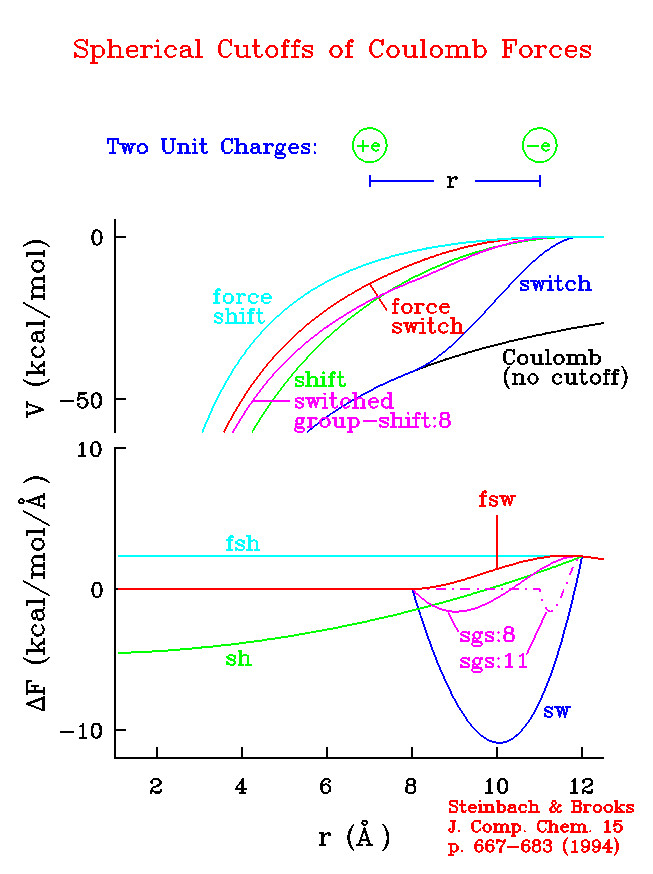
The following figure shows the potential energy of electrostatic interaction for two unit charges (top), as approximated by various methods using a cutoff distance of 12 Å. Also shown is the error in the Coulomb force (bottom) resulting from these approximations. Note the large force errors at long range obtained when using a switching function on the potential energy (switch). This potential-switching method should not be used. The choice made from among the other alternatives available in CHARMM should be made based on the system simulated, as detailed in the following discussion.
 |
|---|
| Potential energy and error in force calculated by some `spherical cutoffs.' |