We now delve into electrostatics to estimate the electrostatic polarization free energy, ![]() , involved in the transfer
of a solute with an arbitrary charge distribution from vacuum to aqueous solution.
, involved in the transfer
of a solute with an arbitrary charge distribution from vacuum to aqueous solution. ![]() is the interaction between the charge distribution and its reaction potential, the potential induced by the charge distribution in the presence of the dielectric
boundary at the solute-solvent interface.
is the interaction between the charge distribution and its reaction potential, the potential induced by the charge distribution in the presence of the dielectric
boundary at the solute-solvent interface.
First, we review some basics. Again, we will focus on important results, and leave most of the mathematical details to textbooks [7]. Don't worry if the equations are unfamiliar; just stay tuned for the punch line.
All problems in electrostatics boil down to the solution of a single equation, Poisson's equation:
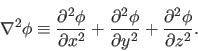
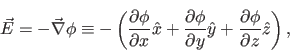
Let's examine two model systems, a point charge and a point dipole, each immersed in a dielectric medium.
In the following two boundary-value problems, we simply state the answer, giving ![]() as a
function of position for all points in space. In these problems, we seek
as a
function of position for all points in space. In these problems, we seek ![]() in regions were there is no charge (
in regions were there is no charge (![]() ).
Thus, we need solutions to the special case of Poisson's equation known as Laplace's equation,
).
Thus, we need solutions to the special case of Poisson's equation known as Laplace's equation,
![]() ,
that satisfy two boundary conditions. First,
,
that satisfy two boundary conditions. First, ![]() must be a continuous function, e.g., at the dielectric boundary.
Second, because there is no `free' charge (charge other than the induced polarization charges) at the dielectric boundary,
the normal component of the electric displacement,
must be a continuous function, e.g., at the dielectric boundary.
Second, because there is no `free' charge (charge other than the induced polarization charges) at the dielectric boundary,
the normal component of the electric displacement,
![]() , will also be continuous at this boundary.
, will also be continuous at this boundary.
First, we model a single ion in solution as a sphere of radius a with a point charge q at its center, immersed in a solvent
of dielectric constant ![]() .
Aside from the point charge at the center, there is nothing inside the solvent-exclusion cavity,
and so the dielectric constant inside is the permittivity of free space
.
Aside from the point charge at the center, there is nothing inside the solvent-exclusion cavity,
and so the dielectric constant inside is the permittivity of free space ![]() . The spherical symmetry
of this system renders it a problem of only one dimension, the distance r from the point charge. The solution is:
. The spherical symmetry
of this system renders it a problem of only one dimension, the distance r from the point charge. The solution is:
One step up in complexity from a point charge is a point dipole. So let's replace the point charge at the center of our solvent-exclusion
sphere with a point dipole ![]() . With this model system we can approximate the solvation energy of a neutral molecule possessing a
permanent dipole moment. Again, the dielectric constant of the solvent is
. With this model system we can approximate the solvation energy of a neutral molecule possessing a
permanent dipole moment. Again, the dielectric constant of the solvent is ![]() , and the dielectric constant inside
the spherical molecule is
, and the dielectric constant inside
the spherical molecule is ![]() . This cylindrically symmetric system has two independent dimensions, the distance r and the
angle
. This cylindrically symmetric system has two independent dimensions, the distance r and the
angle ![]() from the direction of the dipole vector. We get [8]:
from the direction of the dipole vector. We get [8]:
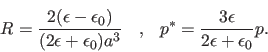
Note that in equations 6 and 7 the potential inside the spherical molecule is a sum of two terms.
In each case, the first term is the potential that would exist in the absence of the dielectric boundary at ![]() , and the second
term is the potential induced in the spherical cavity by the charge distribution's interaction with the dielectric (e.g., the solvent).
The energy of the charge distribution arising from this second term (the reaction potential) gives the electrostatic contribution to
the solvation free energy.
, and the second
term is the potential induced in the spherical cavity by the charge distribution's interaction with the dielectric (e.g., the solvent).
The energy of the charge distribution arising from this second term (the reaction potential) gives the electrostatic contribution to
the solvation free energy.
The energy of a point charge in its reaction potential is one half of the product of the charge and the reaction potential.
The `one half' appears because this is not the energy of a charge in an external electric field. Here, the
charge has contributed to the creation of the field through its electrostatic interactions with the dielectric.
So our continuum model of the solvent predicts that the electrostatic polarization free energy of solvating a spherical ion is
The energy of a dipole in its reaction field (the negative gradient of the reaction potential) is minus
one half of the dot product of the dipole and the reaction field. Again, this is half the energy of a dipole in an external
electric field. The reaction field of the dipole is parallel to the dipole, and we get
Note that both G values are zero if
![]() , i.e., if we haven't changed the dielectric constant of the environment.
, i.e., if we haven't changed the dielectric constant of the environment.
Still and coworkers [10] have proposed the following approximate expression for the free energy of solvent polarization for an
arbitrary charge distribution of N charges:
As shown in the following figure, this GB approximation behaves appropriately in important limiting situations. For N identical,
coincident (![]() ) particles of charge q, it gives the correct Born energy (equation 8, for a single particle of charge
) particles of charge q, it gives the correct Born energy (equation 8, for a single particle of charge ![]() ).
For two charges of equal and opposite sign, it approaches the dipole result (equation 9) at short separation distances, as it should.
For two well separated charges (
).
For two charges of equal and opposite sign, it approaches the dipole result (equation 9) at short separation distances, as it should.
For two well separated charges (
![]() ), it approaches the appropriate energy: the two Born energies plus the energetic
change in the Coulomb interaction between the two charges due to the dielectric medium.
), it approaches the appropriate energy: the two Born energies plus the energetic
change in the Coulomb interaction between the two charges due to the dielectric medium.
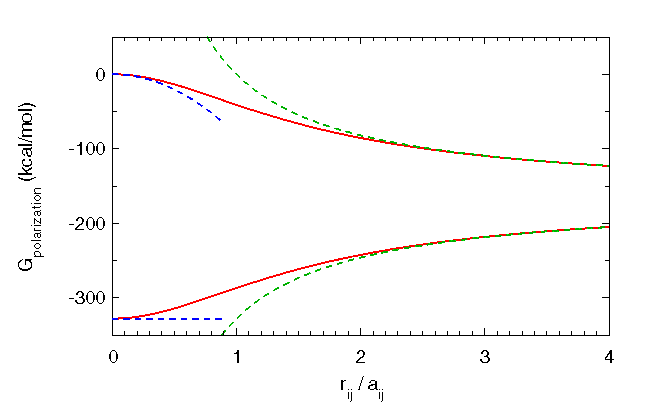 |
|---|
| GB approximation (in red) to solvent polarization energies for two charges of equal radii as a function of separation. The dependence of Born radii on atomic positions is neglected here. Upper curves: Equal and opposite charges with G_dipole (blue) at small separation and Coulomb + Born polarization energies (green) at large separation. Lower curves: Equal charges with G_ion (blue) at zero separation and Coulomb + Born polarization energies (green) at large separation. |