Building on the work of Galileo and others, Newton unveiled his laws of motion in 1686. According to Newton:
I. A body remains at rest or in uniform motion (constant velocity - both speed and direction) unless acted on by a net external force.
II. In response to a net external force, ![]() , a body of mass m accelerates with acceleration
, a body of mass m accelerates with acceleration
![]() .
.
III. If body i pushes on body j with a force ![]() , then body j pushes on body i with a force
, then body j pushes on body i with a force
![]() .
.
For energy-conserving forces, the net force ![]() on particle i is the negative gradient (slope in three dimensions)
of the potential energy with respect to particle i's position:
on particle i is the negative gradient (slope in three dimensions)
of the potential energy with respect to particle i's position:
![]() ,
where
,
where ![]() represents the potential energy of the system as a function of the positions of all N particles,
represents the potential energy of the system as a function of the positions of all N particles, ![]() .
In three dimensions,
.
In three dimensions, ![]() is the vector of length 3 specifying the position of the
is the vector of length 3 specifying the position of the ![]() atom,
and
atom,
and ![]() is the vector of length
is the vector of length ![]() specifying all coordinates.
In the context of simulation, the forces are calculated for energy minimizations and molecular dynamics simulations
but are not needed in Monte Carlo simulations.
specifying all coordinates.
In the context of simulation, the forces are calculated for energy minimizations and molecular dynamics simulations
but are not needed in Monte Carlo simulations.
Classical mechanics is completely deterministic: Given the exact positions and velocities of all particles at a given time, along with
the function ![]() , one can calculate the future (and past) positions and velocities of all particles at any other time. The
evolution of the system's positions and momenta through time is often referred to as a trajectory.
, one can calculate the future (and past) positions and velocities of all particles at any other time. The
evolution of the system's positions and momenta through time is often referred to as a trajectory.
Quantum Mechanics
A number of experimental observations in the late 1800's and early 1900's forced physicists to look beyond Newton's laws of motion for a more general theory. See, for example, the discussion of the heat capacity of solids. It had become increasingly clear that electromagnetic radiation had particle-like properties in addition to its wave-like properties such as diffraction and interference. Planck showed in 1900 that electromagnetic radiation was emitted and absorbed from a black body in discrete quanta, each having energy proportional to the frequency of radiation. In 1904, Einstein invoked these quanta to explain the photo-electric effect. So under certain circumstances, one must interpret electromagnetic waves as being made up of particles. In 1924 de Broglie asserted that matter also had this dual nature: Particles can be wavy.
To make a long and amazing story [1] short, this led to the formulation of Shrödinger's wave equation for matter:
Quantum mechanics is thus not deterministic, but probabilistic. It forces us to abandon the notion of precisely defined trajectories of particles through time and space. Instead, we must talk in terms of probabilities for alternative system configurations.
To clarify these concepts, consider two major successes for the quantum theory, predictions of the discrete energy levels of the harmonic oscillator and the hydrogen atom. Pictured below are the potential energy (solid lines) and the four lowest energy levels (dashed lines) for a one dimensional harmonic oscillator (red) and the three dimensional hydrogen atom (blue). The harmonic oscillator depicted corresponds to a hydrogen atom oscillating at the frequency f = 100/ps and represents one of the highest frequency atomic motions in macromolecules. The energy levels of harmonic oscillators are equally spaced, separated by an energy of hf, or 9.5 kcal/mol for the oscillator shown. The energy gaps for a hydrogen atom oscillating at f = 10/ps are 0.95 kcal/mol, on the order of thermal energy, and so classical mechanics better approximates quantum results (e.g., average energy and motional amplitude) for this slower oscillator.
Excitation of electrons within atoms requires much more energy than excitation of atomic vibrations. Promotion of the hydrogen atom's electron from its ground state to its first excited state requires 235 kcal/mol. Way beyond the reach of thermal energy, this excitation requires the absorption of ultraviolet radiation with a wavelength of 121 nm.
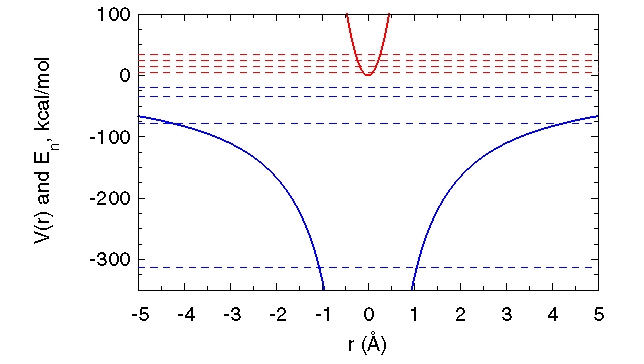 |
|---|
| Potential and four lowest E levels for a harmonic oscillator (red) and the hydrogen atom (blue). |